An impure sample of sodium carbonate,Na2CO3,is titrated with 0.113 M HCl according to the reaction below. 2 HCl(aq) + Na2CO3(aq) 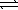 CO2(g) + H2O(
CO2(g) + H2O( 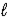 ) + 2 NaCl(aq)
) + 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
A) 0.295%
B) 15.7%
C) 25.5%
D) 51.1%
E) 67.9%
Correct Answer:
Verified
Q55: The solubility of manganese(II)carbonate is 5.4 ×
Q56: The solubility of strontium carbonate (SrCO3)in water
Q57: A solution containing 10.mmol of CO32− and
Q58: A 25.0 mL sample of 0.10 M
Q59: Titration of 0.1615 g of an unknown
Q59: In which of the following solutions would
Q62: What is the minimum mass of Na2CO3
Q63: Given the following equilibrium constants, Zn4IO3)2 Ksp
Q64: Two important biological buffer systems control pH
Q65: What is the molar solubility of Mn(OH)2(s)in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents