Which equation depicts the hydrogen phosphate ion behaving as a Brønsted-Lowry base in water?
A) HPO42-(aq) + H2O( 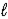 )
) 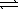 H2PO4-(aq) + OH-(aq)
H2PO4-(aq) + OH-(aq)
B) HPO42-(aq) + OH-(aq) 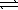 PO43-(aq) + H2O(
PO43-(aq) + H2O( 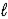 )
)
C) HPO42-(aq) + H2O( 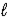 )
)  PO43-(aq) + H3O+(aq)
PO43-(aq) + H3O+(aq)
D) 2 HPO42-(aq) + O2-(aq)  PO43-(aq) + H2O(
PO43-(aq) + H2O( 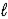 )
)
E) 2 HPO42-(aq) + H2O( 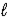 )
) 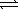 2 H2O(
2 H2O( 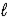 ) + P2O7(s)
) + P2O7(s)
Correct Answer:
Verified
Q6: What is the conjugate base of [Fe(H2O)6]3+(aq)?
A)
Q7: Which of the following substances is never
Q8: Which of the following statements about the
Q9: The autoionization of pure water,as represented by
Q10: Which of the following expressions is not
Q12: Brønsted-Lowry acid-base reactions are restricted to only
Q13: What is the conjugate base of HPO43-
Q14: Which of the following does not behave
Q15: Which equation depicts aqueous hydrogen sulfide behaving
Q16: Which of the following equations shows that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents