The chemical equations below show the reaction of Al(OH)3 as a Lewis acid and as a Lewis base,respectively.
Al(OH)3(s)+ OH-(aq) 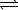 Al(OH)4-(aq)
Al(OH)4-(aq)
Al(OH)3(s)+ 3 H3O+(aq) 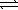 Al3+(aq)+ 3 H2O(
Al3+(aq)+ 3 H2O( 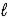 )
)
Substances that can behave as either Lewis acids or bases are called ________ substances.
Correct Answer:
Verified
Q85: When a Lewis acid combines with a
Q86: When a Lewis acid and a Lewis
Q87: A molecule that can behave as either
Q88: All of the following species behave as
Q89: Which of the following molecules or ions
Q90: Write a net ionic equation for the
Q92: Which is the stronger Brønsted-Lowry acid,Fe(H2O)62+ or
Q93: Which of the following species cannot act
Q94: Which of the following is true of
Q95: In the reaction CaO(s)+ CO2(g)→ CaCO3(s),
A) O2-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents