For the reaction 2NO(g) + O2(g) 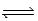 2NO2(g) at 750°C,what is the relationship between Kc and Kp?
2NO2(g) at 750°C,what is the relationship between Kc and Kp?
A) Kc = Kp
B) Kc = Kp × (RT) -1
C) Kc = Kp = 1.0
D) Kc = Kp × (RT) ¾
E) Kc = Kp × (RT) 1
Correct Answer:
Verified
Q11: Write a balanced chemical equation which corresponds
Q12: If the reaction quotient,Q,is greater than K
Q13: Write the expression for Kp for the
Q14: Which of the following is the correct
Q15: Which of the following is the correct
Q17: Given the following chemical equilibrium COCl2(g)
Q18: When gaseous carbon monoxide and hydrogen are
Q19: Which of the following is the correct
Q20: What is the expression for Kc for
Q21: Exactly 1.0 mol N2O4 is placed in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents