Given the following chemical equilibria, N2(g) + O2(g) 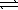 2 NO(g)
2 NO(g)
K1
N2(g) + 3 H2(g) 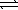 2 NH3(g)
2 NH3(g)
K2
H2(g) + 1/2 O2(g) 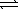 H2O(g)
H2O(g)
K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g) + 5 O2(g) 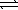 4 NO(g) + 6 H2O(g)
4 NO(g) + 6 H2O(g)
K c
A) 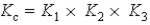
B) 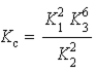
C) 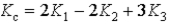
D) 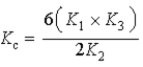
E) 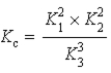
Correct Answer:
Verified
Q38: A 2.5 L flask is filled with
Q39: Which of the following statements about the
Q40: A 3.00-liter flask initially contains 3.00 mol
Q41: The equilibrium constant (Kc)for the decomposition of
Q42: At a given temperature,K = 0.024 for
Q44: Nitrogen and oxygen gases may react to
Q45: Sulfuryl chloride decomposes to sulfur dioxide and
Q46: For the equilibrium N2O4(g) Q47: Nitrosyl bromide decomposes according to the chemical Q48: In an experiment,0.46 mol H2 and 0.46![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents