Which of the following statements is true of the Arrhenius equation?
A) The factor 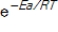 always has a value more than 1.
always has a value more than 1.
B) Ea represents the fraction of molecules having the minimum energy required for a reaction.
C) It can be used to calculate Ea from the temperature dependence of the rate constant.
D) It can be used to calculate the rate constant if 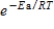 is known.
is known.
E) The factor 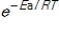 always has a value more than 10.
always has a value more than 10.
Correct Answer:
Verified
Q60: For the overall reaction 2A + B
Q61: Which of the following steps must occur
Q62: Carbon dating may be used to date
Q63: How many mechanistic steps are depicted by
Q64: The pre-exponential,A,in the Arrhenius equation is called
Q66: Circle the catalyst in the following mechanism.
Q67: If a catalyst is present in a
Q68: The _ of an elementary step is
Q69: Termolecular elementary steps are rare.Why?
Q70: Radioactive isotopes decay by _-order kinetics.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents