Henry's Law constant is 0.0013 mol/kg⋅bar and 0.034 mol/kg⋅bar for O2 and CO2 respectively at 25°C.What pressure of CO2 is required to achieve the same solubility as 0.711 bar of O2?
A) 0.0 bar
B) 19.0 bar
C) 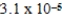 bar
bar
D) 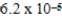 bar
bar
E) 37.0 bar
Correct Answer:
Verified
Q16: What is the definition of molality?
A) moles
Q17: What is the molality of ethanol (C2H5OH)in
Q18: A concentrated hydrochloric acid solution is 37.2%
Q19: A concentrated nitric acid solution has a
Q20: What is the mole fraction of urea,CH4N2O,in
Q22: For the following gas-liquid equilibrium for an
Q23: For the following gas-aqueous liquid equilibrium for
Q24: Which of the following statements is INCORRECT?
A)
Q25: The Henry's law constant for O2 in
Q26: The Henry's law constant for N2 in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents