What is the distance,in atomic radii,along any edge of a body-centered cubic unit cell?
A) 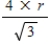
B) 2 × r
C) 4 × r
D) 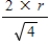
E) r
Correct Answer:
Verified
Q3: Gold (atomic mass 197 g/mol),with an atomic
Q4: Iron crystallizes in a body-centered cubic lattice.If
Q5: Copper crystallizes in a face-centered cubic lattice.The
Q6: What is the length of the diagonal
Q7: Polonium (atomic mass 209.0 g/mol)crystallizes in a
Q9: Palladium crystallizes in a face-centered cubic lattice
Q10: Which one of the following statements is
Q11: Arrange the three common cubic unit cells
Q12: The metal vanadium crystallizes in a body-centered
Q13: Chromium (atomic mass 52.00 g/mol)crystallizes in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents