A sketch of a phase diagram is given below. 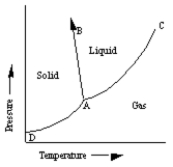 Which statement about this diagram is not true?
Which statement about this diagram is not true?
A) Increasing pressure at constant temperature can melt the solid.
B) Increasing temperature at constant pressure can cause the solid to sublime.
C) Increasing temperature at constant pressure can cause the liquid to vaporize.
D) Increasing pressure at constant temperature can cause deposition of solid from gas.
E) Increasing pressure at constant temperature can cause liquid to freeze.
Correct Answer:
Verified
Q43: What process occurs when the temperature of
Q44: Which of the compounds below is not
Q45: A salt with a 1:1 ratio of
Q46: Above a substance's _ temperature,it is not
Q47: A low-melting solid readily dissolves in water
Q49: The bandgap of ZnTe is 218 kJ/mol.What
Q50: On the phase diagram below,which point corresponds
Q51: Which one of the following elements is
Q52: According to the below phase diagram,what process
Q53: Given the accompanying phase diagram,under what conditions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents