Which intermolecular force or bond is responsible for the density of H2O(s) being less than that of H2O( 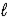 ) ?
) ?
A) London dispersion forces
B) hydrogen bonding
C) ionic bonding
D) covalent bonding
E) dipole/induced dipole forces
Correct Answer:
Verified
Q1: Which one of the following statements is
Q2: As pure molecular solids,which of the following
Q3: Which one of the following sets of
Q5: Hydrogen bonding is present in all of
Q6: Place the following cations in order from
Q7: As pure molecular solids,which of the following
Q8: Arrange H2S,H2Se,and H2Te in order from lowest
Q9: Which of the following statements best describes
Q10: The elements of group 5A,the nitrogen family,form
Q11: Arrange HF,HCl,and HBr in increasing order of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents