What is the total volume of gases produced at 819 K and 1.00 atm pressure when 256 g of ammonium nitrite undergoes the following decomposition reaction? NH4NO2(s) → N2(g) + 2H2O(g)
A) 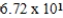 L
L
B) 22.4 L
C) 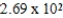 L
L
D) 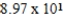 L
L
E) 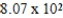 L
L
Correct Answer:
Verified
Q55: What volume of Q56: When 0.5000 grams of an unknown hydrocarbon,CxHy,is Q57: An unknown gaseous hydrocarbon contains 85.63% C.Its Q58: Ammonia gas is synthesized according to the Q59: Aqueous hydrochloric acid reacts with magnesium to Q61: A gaseous mixture containing 1.5 mol Ar Q62: Methane gas,CH4,effuses through a barrier at a Q63: The molar mass of an unknown gas Q64: How long will it take 10.0 mL Q65: The rate of effusion of an unknown![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents