Water can be decomposed by electrolysis into hydrogen gas and oxygen gas.What mass of water must decompose to fill a 3.00 L flask to a total pressure of 2.00 atm at 298 K with a mixture hydrogen and oxygen? (R = 0.08206 L⋅atm/mol⋅K) 2 H2O( 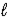 ) → 2 H2(g) + O2(g)
) → 2 H2(g) + O2(g)
A) 1.47 g
B) 2.95 g
C) 4.42 g
D) 6.63 g
E) 8.84 g
Correct Answer:
Verified
Q64: How long will it take 10.0 mL
Q65: The rate of effusion of an unknown
Q66: A pressure of 1.00 atm has a
Q68: Carbon monoxide reacts with oxygen to form
Q70: What is the volume occupied by a
Q71: The partial pressures of CH4,N2,and O2 in
Q73: Calculate the root-mean-square velocity for the O2
Q74: Non-ideal behavior for a gas is most
Q75: In which of the following reactions will
Q90: At STP,as the molar mass of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents