The thermochemical equation for the combustion of butane is givenn below. C4H10(g) + 13/2 O2(g) → 4 CO2(g) + 5 H2O( 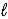 )
)
ΔrH° = -2877 kJ/mol-rxn
What is the enthalpy change for the reaction below?
8 CO2(g) + 10 H2O( 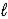 ) → 2 C4H10(g) + 13 O2(g)
) → 2 C4H10(g) + 13 O2(g)
A) +1439 kJ/mol-rxn
B) +2877 kJ/mol-rxn
C) -5754 kJ/mol-rxn
D) -2877 kJ/mol-rxn
E) +5754 kJ/mol-rxn
Correct Answer:
Verified
Q20: Many homes are heated using natural gas.The
Q21: The heat of vaporization of benzene,C6H6,is 30.7
Q22: Given the thermochemical equation 4AlCl3(s)+ 3O2(g)→ 2Al2O3(s)+
Q23: The thermochemical equation for the combustion of
Q24: Commercial cold packs consist of solid ammonium
Q26: Calculate the energy in the form of
Q27: The following reaction of iron oxide with
Q28: 44.0 g of ice at -20.0 °C
Q29: What is the change in internal energy
Q30: What is the minimum mass of ice
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents