What quantity,in moles,of hydrogen is consumed when 179.6 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ? H2(g) + 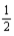 O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
A) 0.6284 mol
B) 0.3142 mol
C) 1.591 mol
D) 1.628 mol
E) 0.3716 mol
Correct Answer:
Verified
Q30: What is the minimum mass of ice
Q31: CaO(s)reacts with water to form Ca(OH)2(aq).If 6.50
Q32: Hydrazine,N2H4,is a liquid used as a rocket
Q33: How much heat is liberated at constant
Q34: At constant pressure and 25°C,what is ΔrH°
Q36: When 10.0 g of KOH is dissolved
Q37: One statement of the first law of
Q38: Which of the following thermodynamic quantities are
Q39: If q = 39 kJ and w
Q40: Calculate ΔU of a gas for a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents