Hydrazine,N2H4,is a liquid used as a rocket fuel.It reacts with oxygen to yield nitrogen gas and water. N2H4( 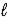 ) + O2(g) → N2(g) + 2 H2O(
) + O2(g) → N2(g) + 2 H2O( 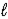 )
)
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
A) -19.4 kJ/mol
B) -25.6 kJ/mol
C) -126 kJ/mol
D) -622 kJ/mol
E) -820.kJ/mol
Correct Answer:
Verified
Q27: The following reaction of iron oxide with
Q28: 44.0 g of ice at -20.0 °C
Q29: What is the change in internal energy
Q30: What is the minimum mass of ice
Q31: CaO(s)reacts with water to form Ca(OH)2(aq).If 6.50
Q33: How much heat is liberated at constant
Q34: At constant pressure and 25°C,what is ΔrH°
Q35: What quantity,in moles,of hydrogen is consumed when
Q36: When 10.0 g of KOH is dissolved
Q37: One statement of the first law of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents