When 50.0 mL of 1.60 M of HCl(aq) is combined with 50.0 mL of 1.70 M of NaOH(aq) in a coffee-cup calorimeter,the temperature of the solution increases by 10.7°C.What is the change in enthalpy for this balanced reaction? HCl(aq) + NaOH(aq) → NaCl(aq) + H2O( 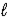 )
)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g⋅°C.
A) -55.8 kJ
B) 55.8 kJ
C) 52.5 kJ
D) -52.5 kJ
E) -27.1 kJ
Correct Answer:
Verified
Q36: When 10.0 g of KOH is dissolved
Q37: One statement of the first law of
Q38: Which of the following thermodynamic quantities are
Q39: If q = 39 kJ and w
Q40: Calculate ΔU of a gas for a
Q42: What is the overall chemical equation that
Q43: Determine ΔrH° for the following reaction,2 NH3(g)+
Q44: A chemical reaction in a bomb calorimeter
Q45: Determine the standard enthalpy of formation of
Q46: A bomb calorimeter has a heat capacity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents