When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) by the following reaction,98.8 kJ of energy is absorbed. Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H2O(g) 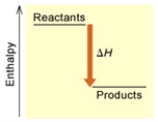
 (A)
(A)
(B)
Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
A) Endothermic; A
B) Endothermic; B
C) Exothermic; A
D) Exothermic; B
E) None of these
Correct Answer:
Verified
Q55: A bomb calorimeter has a heat capacity
Q56: Combustion of 2.14 g of liquid benzene
Q57: Which of the following chemical equations does
Q58: The heat required to convert a solid
Q59: Which of the following has zero standard
Q61: Dry ice converts directly from a solid
Q62: Internal energy and enthalpy are state functions.What
Q63: _ is used to measure the energy
Q64: In a reaction,the values of ∆H and
Q65: Why are you at greater risk from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents