Aspirin (C9H8O4) is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride (M = 102.1 g/mol) . C7H6O3(s) + C4H6O3( 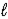 ) → C9H8O4(s) + C2H4O2(
) → C9H8O4(s) + C2H4O2( 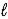 )
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol) can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g
Correct Answer:
Verified
Q15: The compound P4S3 is used in matches.It
Q16: How many moles of Mg3P2(s)can be produced
Q17: If 56.0 g of O2 is mixed
Q18: Potassium hydrogen carbonate decomposes according to the
Q19: Hydrogen peroxide decomposes into oxygen and water.What
Q21: Nitric oxide,NO,is made from the oxidation of
Q22: Consider the fermentation reaction of glucose: C6H12O6
Q23: A compound consists of only C and
Q24: A 1.295 g sample of a hydrocarbon
Q25: Of the following,the only empirical formula is
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents