Which of the following products will form on mixing aqueous solutions of Cu(NO3) 2 and NaOH?
A) Cu(OH) 2(s) ,Na+(aq) ,and NO3−(aq)
B) Cu(OH) 2(s) and NaNO3(s)
C) Cu2(OH) 2(aq) and NaNO3(aq)
D) Cu(OH) 2(aq) and NaNO3(s)
E) Cu(OH) 2(s) ,N2(g) ,and H2O( 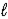 )
)
Correct Answer:
Verified
Q24: Write a balanced equation for the reaction
Q25: Which of the following compounds is a
Q26: Write a balanced chemical equation for the
Q27: Which of the following compounds cannot be
Q28: Which of the following is a weak
Q30: When solutions of barium iodide and lithium
Q31: Nitric acid is the product of the
Q32: Which of the following equation best represents
Q33: If an aqueous solution of _ is
Q34: Write a balanced chemical equation for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents