What is the balanced equation for carbonate ion (CO32-) acting as a Brønsted base in a reaction with water?
A) CO32-(aq) + 3 H2O( 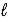 )
)  CO44-(aq) + 2 H3O+(aq)
CO44-(aq) + 2 H3O+(aq)
B) CO32-(aq) + H2O( 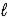 )
)  HCO3-(aq) + OH-(aq)
HCO3-(aq) + OH-(aq)
C) CO32-(aq) + H2O( 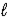 )
) 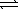 CO2(g) + 2 OH-(aq)
CO2(g) + 2 OH-(aq)
D) CO32-(aq) + 2 H2O( 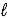 )
) 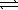 HCO3-(aq) + H3O+(aq)
HCO3-(aq) + H3O+(aq)
E) CO32-(aq) + H2O( 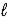 )
) 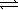 H2CO42-(aq)
H2CO42-(aq)
Correct Answer:
Verified
Q34: Write a balanced chemical equation for the
Q35: What base results when K2O reacts with
Q36: Which of the following is a strong
Q37: When aqueous solutions of calcium iodide,CaI2,and silver
Q38: Which of the following equations best represents
Q40: What is the net ionic equation for
Q41: Hydrocyanic acid,HCN,is a weak acid.Write a net
Q42: What is the oxidation number of each
Q43: What is the oxidation number of P
Q44: Which species in the reaction below undergoes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents