Use the figure below to answer the following questions.
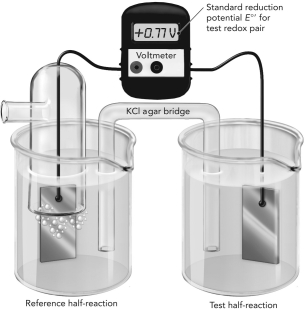 A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
B. Do these oxidants have a higher or lower affinity for electrons compared with H+? Explain how you know this using information from the experiment.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q41: How would an increased level of acetyl-CoA
Q42: High levels of ATP would result in
Q47: How would a high NADH to NAD+
Q56: The reaction catalyzed by is likely
Q58: An in vitro study shows that isocitrate
Q62: Which citrate cycle intermediate is also
Q63: When the citrate cycle is inhibited,
Q64: A low NADH to NAD+ ratio would
Q75: Describe the fate of NADH and FADH2
Q79: The citrate cycle is considered to be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents