Identify the missing particle in the following nuclear equation: 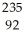 U →
U → 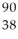 Sr + ? + 2
Sr + ? + 2 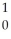 n + 4
n + 4 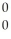 g
g
A) 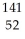 Te
Te
B) 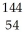 Xe
Xe
C) 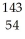 Xe
Xe
D) 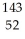 Te
Te
E) 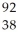 Sr
Sr
Correct Answer:
Verified
Q3: Which of the following nuclides are most
Q41: Nuclides below the valley of stability can
Q53: Identify the missing particle in the following
Q55: What is the correct technique used in
Q56: Above what atomic number are there no
Q57: Stable isotopes with low atomic numbers have
Q60: The radioactive decay of _ is the
Q61: Calculate the mass defect in Fe-56 if
Q62: The following reaction represents what nuclear process?
Q63: The following reaction represents which nuclear process?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents