Complete the following equation of nuclear transmutation. 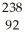 U +
U + 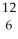 C →
C → 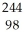 Cf + 6 ________
Cf + 6 ________
A) 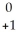 e
e
B) 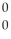 g
g
C) 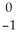 e
e
D) 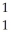 H
H
E) 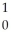 n
n
Correct Answer:
Verified
Q23: Write a nuclear equation to describe the
Q27: The nuclide As-76 has a half-life of
Q77: Fluorine-18 undergoes positron emission with a half-life
Q78: Calculate the mass defect in Ni-59 if
Q81: Which of the following elements would be
Q83: Complete the following equation of transmutation.
Q84: Strontium-90 is a byproduct in nuclear reactors
Q85: If we start with 1.000 g of
Q105: Neptunium-239 has a half-life of 2.35 days.How
Q106: What percentage of a radioactive substance remains
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents