Multiple Choice
How much copper, in g, can be plated from a solution of 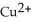 if 3.14 A is applied for 45 minutes?
if 3.14 A is applied for 45 minutes?
A) 2.79 g
B) 1.85 g
C) 11.2 g
D) 0.283 g
E) 7.64 g
Correct Answer:
Verified
Related Questions
Q43: What mass of silver can be plated
Q50: What mass of aluminum can be plated
Q90: Given that E°red = -1.66 V for
Q92: A galvanic cell consists of a Zn2+/
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents