How much chromium, in g, can be plated from a solution of 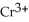 if 2.72 A is applied for 15 minutes?
if 2.72 A is applied for 15 minutes?
A) 1.32 g
B) 6.51 × 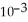 g
g
C) 0.937 g
D) 0.440 g
E) 4.67 g
Correct Answer:
Verified
Q57: Nickel can be plated from aqueous solution
Q84: In the presence of which of the
Q86: What is the shorthand notation that represents
Q90: Given that E°red = -1.66 V for
Q92: A galvanic cell consists of a Zn2+/
Q104: What is the balanced chemical equation for
Q114: Using the following standard reduction potentials,
Fe3+(aq)+ e-
Q117: What is the balanced equation for the
Q118: For the galvanic cell reaction,expressed below using
Q120: A galvanic cell consists of one half-cell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents