The electrolysis of molten 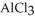 for 4.25 hr with an electrical current of 25.0 A produces
for 4.25 hr with an electrical current of 25.0 A produces  of aluminum metal.
of aluminum metal.
A) 9.90 × 10-3
B) 107
C) 321
D) 1.32
E) 35.7
Correct Answer:
Verified
Q62: Match the following.
-Q > K
A)Ecell = E°cell
B)Ecell
Q62: Match the following.
-Q > K
A)Ecell = E°cell
B)Ecell
Q69: Match the following.
-Q = 1
A)Ecell = E°cell
B)Ecell
Q107: The gas OF2 can be produced from
Q108: The standard emf for the cell using
Q110: The standard cell potential (E°) of a
Q113: Match the following.
-ΔrG° < 0
A)Ecell = E°cell
B)Ecell
Q113: Match the following.
-ΔrG° < 0
A)Ecell = E°cell
B)Ecell
Q125: How long must a constant current of
Q127: How many grams of nickel metal are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents