Use the following thermodynamic values to calculate Δr 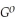 .
.
Δr
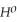 = -95 kJ
= -95 kJ 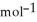 , Δr
, Δr
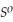 = -157 J
= -157 J 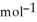 , T = 298 K
, T = 298 K
A) -48 kJ 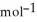
B) -68 kJ 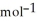
C) + 39 kJ 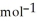
D) -157 kJ 
E) +142 kJ 
Correct Answer:
Verified
Q27: Place the following in order of decreasing
Q29: Place the following in order of increasing
Q31: Place the following in order of increasing
Q32: Above what temperature does the following reaction
Q33: Below what temperature does the following reaction
Q34: Place the following in order of increasing
Q37: Use the following thermodynamic values to calculate
Q39: Place the following in order of decreasing
Q40: For the following example, what is true
Q41: Given the following equation, N2O(g) + NO2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents