Use the following thermodynamic values to calculate Δr 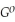 .
.
Δr
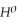 = +95 kJ
= +95 kJ 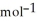 , Δr
, Δr
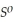 = -157 J
= -157 J 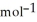 , T = 298 K
, T = 298 K
A) -48 kJ 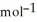
B) -68 kJ 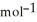
C) +39 kJ 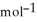
D) -157 kJ 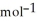
E) +142 kJ 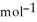
Correct Answer:
Verified
Q27: Place the following in order of decreasing
Q29: Place the following in order of increasing
Q32: Above what temperature does the following reaction
Q33: Below what temperature does the following reaction
Q34: Place the following in order of increasing
Q36: Use the following thermodynamic values to calculate
Q39: Place the following in order of decreasing
Q40: For the following example, what is true
Q41: Given the following equation, N2O(g) + NO2(g)
Q42: What is the change in Gibbs energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents