Which one of the following would be expected to have the lowest standard molar entropy, S°, at 25 °C?
A) C10H22(s)
B) C10H22(l)
C) C14H30(s)
D) C14 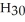 OH(l)
OH(l)
Correct Answer:
Verified
Q98: Which of the following is TRUE if
Q99: Which of the following reactions will have
Q100: Calculate ΔrG for the evaporation of methanol
Q101: Determine the Q of a reaction at
Q102: Phosphorus and chlorine gases combine to produce
Q104: Determine the temperature of a reaction if
Q106: Determine the Q of a reaction at
Q107: Match the following.
-Q = K
A)standard state
B)equilibrium
C)ΔrG >
Q108: The value of ΔfG° at 400.0 °C
Q123: Under which of the following conditions would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents