An acid solution is 0.100 mol L-1 in HCl and 0.200 mol L-1 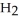
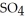 . What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?
. What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?
A) 0.700 L
B) 1.98 L
C) 1.67 L
D) 1.02 L
E) 0.250 L
Correct Answer:
Verified
Q12: When titrating a weak monoprotic acid with
Q48: Which of the following compounds will have
Q56: Identify the most commonly used indicator among
Q58: What is the pH of a solution
Q59: A 1.0 L buffer solution is 0.050
Q60: A 100.0 mL sample of 0.18 mol
Q62: A 100.0 mL sample of 0.20 mol
Q63: A 100.0 mL sample of 0.20 mol
Q64: Identify the salts that are in hard
Q66: A 100.0 mL sample of 0.20 mol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents