A 25.0 mL sample of 0.150 mol L-1 formic acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH at the equivalence point? The 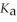 of formic acid is 1.8 × 10-4.
of formic acid is 1.8 × 10-4.
A) 11.38
B) 10.26
C) 5.69
D) 8.31
E) 7.00
Correct Answer:
Verified
Q117: What is the pH of a buffer
Q129: What is the pH of a solution
Q130: Calculate the percent ionization of nitrous acid
Q131: What is the pH of a solution
Q132: What is the hydronium ion concentration in
Q133: What is the pH of a buffer
Q135: What is the pH of a buffer
Q137: How many millilitres of 0.120 mol L-1
Q138: What is the pH of a solution
Q139: Calculate the percent ionization of nitrous acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents