A 25.0 mL sample of 0.150 mol L-1 hydrofluoric acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH before any base is added? Hydrofluoric acid is monoprotic. The 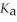 of hydrofluoric acid is 3.5 × 10-4.
of hydrofluoric acid is 3.5 × 10-4.
A) 2.33 × 10-3
B) 2.63
C) 3.46
D) 7.07 × 10-3
E) 2.15
Correct Answer:
Verified
Q118: A sample contains Ba3(PO4)2, CdS, AgCl, NH4Cl,
Q119: A solution contains 0.021 mol L-1 Cl⁻
Q120: A solution containing CaCl2 is mixed with
Q121: What volume of 5.00 × 10-3 mol
Q122: What is the approximate pH at the
Q124: How many millilitres of 0.0890 mol L-1
Q125: What is the [CH3COO-]/[CH3COOH] ratio necessary to
Q126: What is the pH of a solution
Q127: A 25.0 mL sample of 0.150 mol
Q128: What is the approximate pH at the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents