What is the pH of a solution made by mixing 30.00 mL of 0.10 mol L-1 acetic acid (CH3COOH) with 50.00 mL of 0. 100 mol L-1 KOH? Assume that the volumes of the solutions are additive. Ka = 1.8 × 10-5 for 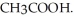
A) 8.26
B) 9.26
C) 11.13
D) 12. 40
Correct Answer:
Verified
Q151: In which of the following solutions would
Q152: What is the molar solubility of Mg(OH)2
Q153: Calculate the Ksp for silver carbonate if
Q154: What is the pH of a solution
Q155: Formic acid (HCOOH, Ka = 1.8 ×
Q157: What is the molar solubility of silver
Q158: Sodium hypochlorite, NaOCl, is the active ingredient
Q159: Calculate the molar solubility of thallium chloride
Q160: What is the molar solubility of calcium
Q161: Predict the number of equivalence point(s) in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents