At 900.0 K, the equilibrium constant (Kp) for the following reaction is 0.345 bar-1: 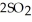 +
+ 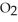 (g) →
(g) → 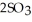 (g) At equilibrium, the partial pressure of
(g) At equilibrium, the partial pressure of 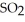 is 35.0 bar and that of
is 35.0 bar and that of 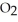 is 15.9 bar. The partial pressure of
is 15.9 bar. The partial pressure of 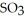 is ________ bar.
is ________ bar.
A) 82.0
B) 192
C) 4.21 × 10-3
D) 55.3
E) 6.24 × 10-3
Correct Answer:
Verified
Q105: At a certain temperature,Kc equals 1.40 ×
Q116: Cyclohexane (C6H12)undergoes a molecular rearrangement in the
Q125: Why aren't solids or liquids included in
Q130: Explain dynamic equilibrium.Use the generic reaction A(g)⇌
Q135: Dinitrogen tetroxide partially decomposes according to the
Q137: Match the following.
-Q < K
A)reverse reaction is
Q137: How is the reaction quotient different from
Q137: Match the following.
-Q < K
A)reverse reaction is
Q138: The equilibrium constant, Kp, equals 3.40 for
Q144: Why is a thermodynamic equilibrium constant unitless?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents