Phosphorus trichloride and phosphorus pentachloride equilibrate in the presence of molecular chlorine according to the following reaction: 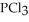 (g) +
(g) + 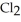 (g) →
(g) → 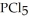 (g) An equilibrium mixture at 450 K contains
(g) An equilibrium mixture at 450 K contains 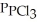 = 0.124 bar,
= 0.124 bar, 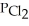 = 0.157 bar, and
= 0.157 bar, and 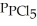 = 1.30 bar. What is the value of Kp at this temperature?
= 1.30 bar. What is the value of Kp at this temperature?
A) 66.8 bar-1
B) 4.63 bar-1
C) 2.53 × 10-2 bar-1
D) 1.50 × 10-2 bar-1
E) 1.03 bar-1
Correct Answer:
Verified
Q102: At a certain temperature,nitogen and hydrogen react
Q121: Match the following.
-K << 1
A)reverse reaction is
Q124: Kc is 1.67 × 1020 mol3 L-3
Q125: The Q126: At a certain temperature the equilibrium constant, Q128: An equilibrium mixture of CO, O2, and Q131: The equilibrium constant, Kp, equals 3.40 at Q132: The decomposition of ammonia is 2NH3(g) → Q133: Phosphorus pentachloride decomposes to phosphorus trichloride at Q134: For the isomerization reaction: butane ⇌ isobutane![]()
Kp
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents