Which of the following represents the equation for a zero-order half-life?
A) t 1/2 = 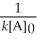
B) t 1/2 = 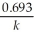
C) t 1/2 = 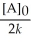
D) t 1/2 = 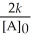
E) t 1/2 = 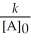
Correct Answer:
Verified
Q26: The rate constant for the first-order decomposition
Q39: The half-life for the decay of radium
Q66: The first-order decomposition of N2O at 1000
Q67: Derive an expression for a "1/4-life" for
Q68: The rate constant for a zero-order reaction
Q69: The rate constant for a second-order reaction
Q71: The first-order decomposition of N2O5 at 328
Q72: How many half-lives are required for the
Q73: Derive an expression for a "1/3-life" for
Q74: The first-order decomposition of cyclopropane has a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents