Derive an expression for a "1/4-life" for a first-order reaction.
A) 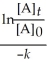
B) 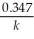
C) 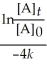
D) 
E) 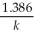
Correct Answer:
Verified
Q26: The rate constant for the first-order decomposition
Q54: How many half-lives are required for the
Q62: The first-order decay of radon has a
Q63: The rate constant for a first-order reaction
Q66: The first-order decomposition of N2O at 1000
Q68: The rate constant for a zero-order reaction
Q69: The rate constant for a second-order reaction
Q70: Which of the following represents the equation
Q71: The first-order decomposition of N2O5 at 328
Q72: How many half-lives are required for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents