The rate of disappearance of HBr in the gas phase reaction 2HBr(g) → 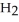 (g) +
(g) + 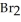 (g)
(g)
Is 0.311 mol L-1 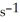 at 150 °C. The rate of appearance of
at 150 °C. The rate of appearance of 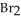 is ________ mol L-1
is ________ mol L-1 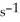 .
.
A) 1.61
B) 0.0967
C) 0.558
D) 0.622
E) 0.156
Correct Answer:
Verified
Q87: The decomposition of dinitrogen pentoxide is described
Q88: For a reaction that follows the general
Q100: For a reaction that follows the general
Q102: For a particular first-order reaction,it takes 24
Q126: What is the overall reaction order for
Q127: The isomerization of methylisonitrile to acetonitrile
Q129: What is one of the conditions that
Q130: The combustion of ethylene proceeds by the
Q131: Identify a heterogeneous catalyst.
A) CFCs with ozone
B)
Q132: The decomposition of ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents