A particular first-order reaction has a rate constant of 1.35 × 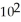
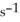 at 25.0 °C. What is the magnitude of k at 75.0 °C if
at 25.0 °C. What is the magnitude of k at 75.0 °C if 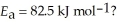
A) 1.61 × 104 s-1
B) 646 s-1
C) 1.11 × 10117 s-1
D) 6.19 × 10-5 s-1
E) 1.36 × 102 s-1
Correct Answer:
Verified
Q83: The decomposition of dinitrogen pentoxide is described
Q106: The isomerization reaction,CH3NC → CH3CN,is first order
Q112: The second-order reaction 2 Mn(CO)5 → Mn2(CO)10
Q117: The first-order reaction,2 N2O(g)→ 2 N2(g)+ O2(g),has
Q141: Match the following.
-t1/2
A)reaction order
B)frequency factor
C)activation energy
D)rate
Q142: For the first-order reaction, 2N2O(g) → 2N2(g)
Q143: Match the following.
-n, in Rate = k[A]n
A)reaction
Q144: In aqueous solution, hypobromite ion, BrO-, reacts
Q147: The first-order reaction, SO2Cl2 → SO2 +
Q150: Match the following.
-k
A)reaction order
B)frequency factor
C)activation energy
D)rate constant
E)half-life
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents