At 20 °C, a 2.72 mol L-1 aqueous solution of ammonium chloride has a density of 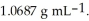 What is the molality of ammonium chloride in the solution? The formula weight of
What is the molality of ammonium chloride in the solution? The formula weight of 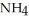 Cl is
Cl is 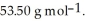
A) 0.339 mol kg-1
B) 2.72 mol kg-1
C) 0.393 mol kg-1
D) 13.6 mol kg-1
E) 2.95 mol kg-1
Correct Answer:
Verified
Q101: A solution is 2.25% by weight NaHCO3.How
Q107: What is the weight percent of vitamin
Q111: What molality of pentane is obtained by
Q116: To make a 0.500 mol L-1 solution,one
Q117: To make a 2.00 mol kg-1 solution,one
Q119: A solution of LiCl in water is
Q124: A solution is prepared by dissolving 16.4
Q125: A solution of LiCl in water has
Q132: What volume of a 0.716 mol L-1
Q155: How much water must be added to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents