A solution is prepared by adding 40.00 g of lactose (milk sugar) to 110.0 g of water at 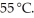 The partial pressure of water above the solution is ________ Torr. The vapour pressure of pure water at 55 °C is 0.1553 bar. The MW of lactose is
The partial pressure of water above the solution is ________ Torr. The vapour pressure of pure water at 55 °C is 0.1553 bar. The MW of lactose is 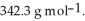
A) 118.7
B) 8.268
C) 19.08
D) 2.188
E) 114.3
Correct Answer:
Verified
Q121: What is the expected freezing point of
Q141: Explain why water does not dissolve in
Q144: Calculate the freezing point of a solution
Q145: What happens to a supersaturated solution of
Q145: Match the following.
-0.020 mol kg-1 NH4Cl
A)solution of
Q146: Why isn't pentanol (CH3CH2CH2CH2CH2OH)very soluble in water?
Q149: Calculate the freezing point of a solution
Q150: A solution is prepared by dissolving 21.0
Q152: At 20 °C, a 0.389 mol L-1
Q153: At a given temperature the vapour pressures
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents