Vanadium crystallizes in a body-centred cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium if the edge length of a bcc structure is 4r/ 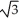 .
.
A) 3.06 g cm-3
B) 12.2 g cm-3
C) 6.11 g cm-3
D) 2.77 g cm-3
E) 8.46 g cm-3
Correct Answer:
Verified
Q9: Identify the type of solid for argon.
A)
Q68: Which of the following is considered a
Q79: Which of the following is considered an
Q93: What is the packing efficiency in face-centred
Q95: What is the packing efficiency in body-centred
Q98: Determine the radius of an Al
Q99: Why is water an extraordinary substance?
A) Water
Q100: Give the coordination number for a body-centred
Q101: Calculate the total quantity of heat required
Q102: Based on the figure above, the boiling
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents