The fluorocarbon 
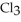
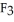 has a normal boiling point of 47.6 °C. The specific heats of
has a normal boiling point of 47.6 °C. The specific heats of 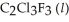 and
and 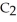
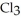
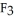 (g) are 0.91 J g-1 °C-1 and 0.67 J g-1 °C-1, respectively. The heat of vaporization of the compound is 27.49 kJ mol-1. The heat required to convert 50.0 g of the compound from the liquid at
(g) are 0.91 J g-1 °C-1 and 0.67 J g-1 °C-1, respectively. The heat of vaporization of the compound is 27.49 kJ mol-1. The heat required to convert 50.0 g of the compound from the liquid at 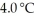 to the gas at 70.0 °C is ________ kJ.
to the gas at 70.0 °C is ________ kJ.
A) 10.07
B) 2742
C) 30.22
D) 9.86
E) 4.60
Correct Answer:
Verified
Q100: Give the coordination number for a body-centred
Q101: Calculate the total quantity of heat required
Q102: Based on the figure above, the boiling
Q103: Ethyl chloride, C2H5Cl, is used as a
Q104: Based on the figure above, the boiling
Q106: The heat of vaporization of water at
Q107: Which of the following compounds exhibits hydrogen
Q110: How much heat is released when 105
Q111: The normal boiling point for H2Se
Q117: Which has the smallest dipole-dipole forces?
A)CH3Cl
B)HBr
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents