Ethanol ( 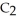
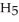 OH) melts at -114 °C. The enthalpy of fusion is 5.02 kJ mol-1. The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1, respectively. How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -145 °C to liquid ethanol at -60 °C?
OH) melts at -114 °C. The enthalpy of fusion is 5.02 kJ mol-1. The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1, respectively. How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -145 °C to liquid ethanol at -60 °C?
A) 6.58
B) 9.96
C) 8.88
D) 3859
E) -13.56
Correct Answer:
Verified
Q101: Which of the following compounds exhibits only
Q108: Lithium crystallizes in a body-centred cubic structure.What
Q112: Which of the following compounds has the
Q113: What is the edge length of a
Q114: In liquid ethanol, CH3CH2OH
Which intermolecular forces
Q116: The enthalpy change for converting 10.0 g
Q118: Which of the following substances should have
Q119: The enthalpy change for converting 1.00 mol
Q124: Define volatile.
Q146: Which is expected to have the largest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents