Which of the following processes is endothermic?
A) K⁺(g) + I⁻(g) → KI(s)
B) 2Br(g) → Br2(g)
C) Ca(s) → Ca(g)
D) 2Na(s) + 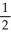 O2(g) → Na2O(s)
O2(g) → Na2O(s)
E) None of the above processes is endothermic.
Correct Answer:
Verified
Q29: Place the following elements in order of
Q43: Rank the following molecules in decreasing bond
Q56: Place the following in order of increasing
Q65: Choose the bond below that is the
Q68: Use the bond energies provided to estimate
Q72: Which of the following processes is exothermic?
A)
Q74: Use the bond energies provided to estimate
Q75: Which of the following processes is exothermic?
A)
Q77: Which of the following processes is endothermic?
A)
Q78: Use the bond energies provided to estimate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents