A 6.55 g sample of aniline (C6H5NH2, molar mass = 93.13 g 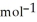 ) was combusted in a bomb calorimeter. If the temperature rose by 32.9 °C, use the information below to determine the heat capacity of the calorimeter. 4C6H5NH2(l) + 35O2(g) → 24CO2(g) + 14H2O(g) + 4NO2(g)
) was combusted in a bomb calorimeter. If the temperature rose by 32.9 °C, use the information below to determine the heat capacity of the calorimeter. 4C6H5NH2(l) + 35O2(g) → 24CO2(g) + 14H2O(g) + 4NO2(g)
ΔrU= -3.20 × 103 kJ mol-1
A) 97.3 kJ 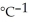
B) 38.9 kJ 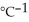
C) 5.94 kJ 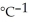
D) 6.84 kJ 
E) 12.8 kJ 
Correct Answer:
Verified
Q28: A 2.38 g sample of phenol (C6H6O,
Q29: A chemist wishes to calibrate a bomb
Q30: Calculate the internal energy change, ΔrU, for
Q31: Calculate the internal energy change, ΔrU, for
Q32: A 21.8 g sample of ethanol (C2H5OH)
Q34: The change in enthalpy for any process
Q35: A chemist wishes to calibrate a bomb
Q36: A balloon is inflated from 0.0100 L
Q37: Calculate the change in internal energy (ΔU)
Q38: An 8.21 g sample of glycerol (C3H8O3,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents