Which of the following statements is TRUE?
A) State functions do not depend on the path taken to arrive at a particular state.
B) 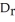 U can be determined using constant volume calorimetry.
U can be determined using constant volume calorimetry.
C) Energy is neither created nor destroyed, excluding nuclear reactions.
D) 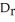 H can be determined using constant pressure calorimetry.
H can be determined using constant pressure calorimetry.
E) All of the above are true.
Correct Answer:
Verified
Q56: Using the following equation for the combustion
Q57: According to the following reaction, how much
Q58: Which of the following processes is endothermic?
A)
Q59: A 12.8 g sample of ethanol (C2H5OH)
Q60: A bomb calorimeter with a heat capacity
Q62: Calculate the heat transfer, in J, when
Q63: An unknown metal alloy, mass = 36.1
Q64: According to the following thermochemical equation, what
Q65: Calculate the initial temperature of 21.8 g
Q66: Calculate the final temperature of 82.1 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents