Use the standard reaction enthalpies given below to determine 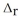 H° for the following reaction: P4(g) + 10Cl2(g) → 4PCl5(s)
H° for the following reaction: P4(g) + 10Cl2(g) → 4PCl5(s) 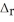 H° = ?
H° = ?
Given:
PCl5(s) → PCl3(g) + Cl2(g) 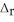 H°= +157 kJ
H°= +157 kJ
P4(g) + 6Cl2(g) → 4PCl3(g) 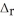 H° = -1207 kJ
H° = -1207 kJ
A) -1835 kJ mol-1
B) -1364 kJ mol-1
C) -1050 kJ mol-1
D) -1786 kJ mol-1
E) -2100 kJ mol-1
Correct Answer:
Verified
Q80: According to the following thermochemical equation, what
Q81: Calculate the enthalpy of solution for the
Q82: An unknown metal alloy, specific heat capacity
Q83: Calculate the final temperature of a 38.1
Q84: A piece of gold with a mass
Q86: A piece of uranium with a mass
Q87: 9.26 g of an unknown solid is
Q88: Two solutions, initially at 24.69 °C, are
Q89: A piece of iron (mass = 25.0
Q90: Calculate the mass of sodium cyanate (NaOCN,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents