When 50.0 mL of 0.400 mol L-1 Ca(NO3) 2 is added to 50.0 mL of 0.800 mol L-1 NaF, CaF2 precipitates, as shown in the net ionic equation below. The initial temperature of both solutions is 24.0 °C. Assuming that the reaction goes to completion, and that the resulting solution has a mass of 100.00 g and a specific heat of 4.18 J 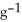
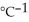 , calculate the final temperature of the solution. Ca2+(aq) + 2F-(aq) → CaF2(s)
, calculate the final temperature of the solution. Ca2+(aq) + 2F-(aq) → CaF2(s) 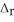 H = -11.5 kJ
H = -11.5 kJ
A) 23.45 °C
B) 24.55 °C
C) 25.10 °C
D) 25.65 °C
Correct Answer:
Verified
Q123: Why are the standard heats of formation
Q138: Give the temperature and pressure for the
Q151: Match the following.
-energy associated with the temperature
Q153: Explain the difference between ΔH and ΔU.
Q154: Match the following.
--w
A)energy flows out of the
Q156: Match the following.
-energy associated with the position
Q157: Match the following.
-energy associated with the motion
Q158: The standard state of sulfur is _.
Q159: When 2.50 g of Ba(s) is added
Q161: The standard state of carbon is _.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents