If 0.3781 g of a gas with a molecular weight of 18.015 g 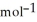 is in a ridged 5.00 L container at 384 K, calculate the final pressure. Assume ideal behaviour.
is in a ridged 5.00 L container at 384 K, calculate the final pressure. Assume ideal behaviour.
A) 0.682 bar
B) 0.109 bar
C) 0.0867 bar
D) 0.132 bar
E) 0.0963 bar
Correct Answer:
Verified
Q36: Which of the following samples has the
Q38: Place the following gases in order of
Q44: Calculate the mass of nitrogen dioxide, NO2
Q45: The density of a gas is 1.41
Q48: What pressure will 2.6 × 1023 molecules
Q50: The density of a gas is 1.146
Q51: Determine the density of CO2 gas at
Q52: How many molecules of CO2 are contained
Q53: Give the temperature and pressure at STP.
A)
Q54: Which of the following samples will have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents